Single Molecule Science—the ability to observe and track individual molecules and monitor molecular interactions in living cells—heralds a revolution in biology.
It’s a collaboration between biologists, physicists and engineers that feeds off the capacity of new microscopes and other technology to identify single molecules and analyse their behaviour in intact samples.
It has emerged from developing, using and combining technologies such as single-molecule fluorescence and super resolution microscopy, atomic force microscopy, optical and magnetic tweezers, new sensors and more sophisticated computing and mathematical techniques.
Single Molecule Science presents us with a new way of understanding life processes from the bottom up, following the paths and changes of many different molecules as they move through the cell, form complexes with other molecules, and influence cell function.
And the more we know about molecular behaviour in cells and organs, the better we are able to solve medical problems such as those involving DNA replication and repair and how they related to the origin of cancers; signalling networks and inhibitors that regulate the immune system; and protein transport, for example in neuroscience.
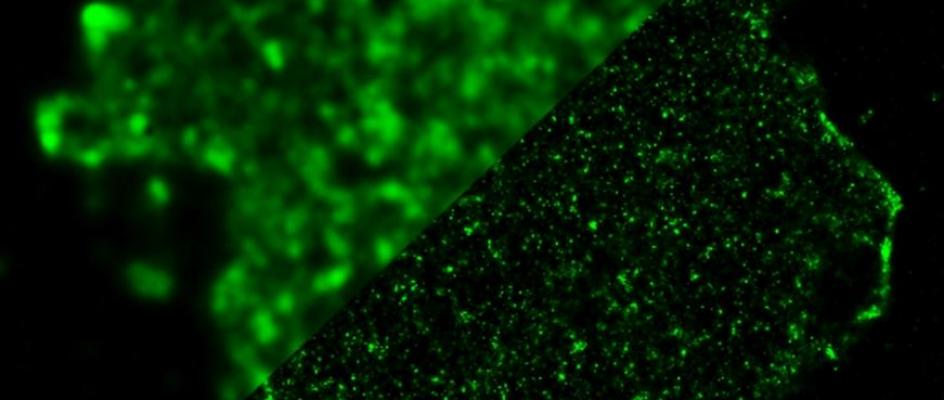
Image: Seeing clearly ... a standard image of the interior of a T-Cell (upper left) versus the super resolution image..
Observing life from the bottom up
Ever since 1953, when James Watson, Francis Crick—with data from Rosalind Franklin—determined the molecular structure of DNA and showed how it replicates, it has been indisputable that cells, organisms and life itself result from the interactions of biomolecules, such as proteins, carbohydrates, fats and nucleic acids. In fact, the structure and function of each and every cell is founded on the interactions of many millions of molecules that happen to be in the right place at the right time to assemble into cellular structures and initiate the activities of life.
Although we talk of microscopic cellular structures—such as the internal skeleton and membranes, and of cellular activities, such as signalling and internal transport—as if they were similar to our own macroscopic world, they are really quite different.
The molecular scene is much more fluid and chaotic.
But it’s the molecules themselves that impose order on this chaos. They come together independently and organise themselves into the molecular complexes at the heart of cellular structure and function. The turnover of these self-assembled complexes can be very rapid. They form, play a role in cellular function and then fall apart. And the same molecule or component can be part of several different complexes with very different functions.
It is now also recognised that single molecules behave quite differently in the complex architecture of cells and tissue than they do in solution or on the artificial surfaces found in the laboratory. This is because the structural elements—the cytoskeleton and membranes—are exceedingly complex themselves and are not static.
In fact, single-molecule behaviours are deeply integrated into the cellular architecture and vice versa. Cellular architecture itself emerges from single-molecule reactions.
By observing and tracking single molecules we can see important but rare events that are normally lost. We now need to link the molecular behaviour to cellular outcomes and physiological responses so that we have seamless knowledge from the molecular to macroscopic world, and from the cause to the presentation of disease.
Working with single molecules for better health
The more we know about how molecules move and interact in time and space, the better we are able to work in collaboration with the responses of our bodies, and particularly the immune system, to solve medical problems.
Take T cell activation and the immune response, for example. T cells are among the basic components, the foot soldiers, of our body’s defence or immune system. They sense minute amounts of the protein fragments or peptides generated by disease causing organisms and can respond by triggering an immune response.
The decision of a T cell, whether to activate or not is determined by a signalling network inside the cell. In this decision-making network, information is encoded in the frequency and duration of the interactions of its components. Hence a drug that inactivates a component in the network and stops all its interaction can lead to the complete collapse of the system which can severely compromise immunity.
On the other hand, drugs that simply modulate or tune interactions at key junctions in the network to skew the outcome can, for example, de-sensitise T cells without limiting immune responses to pathogens altogether. This could be particularly beneficial to patients with an autoimmune condition, where the immune system is sensitive to and attacks the body itself. If we can identify the molecular rules by which the T cell signalling network operates, we might well be able to design drugs that work with T cells to re-balance the immune system, rather than fighting against it.
Another example is cancer immunotherapy, a strategy to harness the body’s immune system to combat tumours. We can already build T cell receptors which will detect cancers with super-physiological affinity. However, when these ‘super-receptors’ are put into human T cells, their responsiveness is poor. This is because T cell responses are not the sum of the individual parts.
Single molecule science should allow us to work out exactly how the parts work together so that these ‘super-receptors’ can be properly integrated into the T cell signalling network. Only then can T cells use this new man-made tool to sense and combat cancer.
This way of thinking can be extended to many other medical areas, such as DNA replication and repair, understanding the origin of cancers and protein transport particularly in neuroscience.
SMS – a high-tech science
The new science of single molecules is a discipline of cell biology, biophysics and physiology, but it also incorporates chemistry, physics, mathematics and engineering and approaches from nanotechnology, biotechnology and nano-fabrication.